StudyWorks
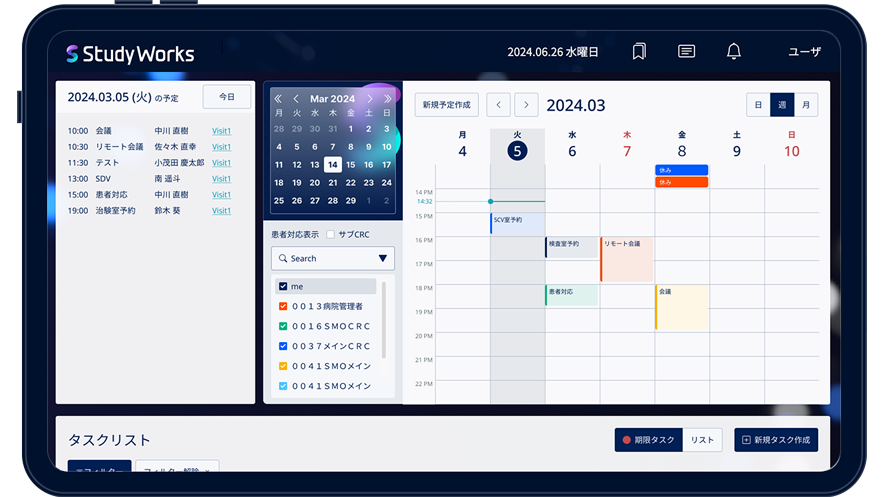
Study Works is a cutting-edge platform designed from the ground up based on insights from CRCs (Clinical Research Coordinators) and CRAs (Clinical Research Associates). It revolutionizes trial management by offering a seamless, one-stop solution for coordinating with stakeholders—ranging from pharmaceutical companies and CROs to patients and various departments within trial sites. By integrating and streamlining the vast array of trial management tasks through Study Works, we dramatically cut down on administrative overhead and enhance operational efficiency.

| Key Features | Feature Overview | Benefits for Sponsors and CROs |
|---|---|---|
| Cost aggregation | Automatic aggregation function for clinical trial expenses such as subject burden reduction expenses and research expenses | You can see a list of when and when expenses were incurred, making it easier to check billing details. |
| Task management function | Task trial at each visit its task allocation |
By sharing and assigning tasks that require deadline management with hospital staff, you can reduce human errors and share requests. |
| Subject visit schedule management | Subject visit schedule management | You can easily check the date of your next visit. You can mutually check whether the visit is within Allowance. |
| Sharing candidate status/progress information | Confirming the number of candidates before obtaining consent Sharing and summarizing progress information and consultation |
You will be able to obtain information about in-hospital candidates and discuss action plans with the CRC. *We cannot confirm any personal information. |
| Reception list | List management of subject visit schedule | This function allows you to see a list of which tests the subjects are in, when they will come to the hospital, and who will be in charge. |
| SDV reservation function | SDV room reservation calendar function | You can check room availability and manage SDV reservations smoothly, reducing the hassle of communication. |
| File sharing function | Shared storage for files | Since it can be used as storage, file sharing among related parties can be done smoothly. |
| Chat function | Chat function improves work efficiency and speeds communication | Real-time information sharing facilitates clinical trial projects and strengthens collaboration across teams. |
| Notice function | Posting and distribution of notifications from within the hospital (confirmation function) | You can also receive information from within the hospital and manage whether the information has been confirmed. |
Unified One-Stop Management of All CRC-Centric Operations
Seamlessly integrate and manage CRC-focused activities—such as clinical trial operations, cost control, progress tracking, resource management, task coordination, and sponsor interactions—while streamlining communication with CRAs, all within a unified, innovative platform.
For Sponsors and CRAs:
・Move Beyond Excel
・Improve Candidate and Site Management
・Automate Reports
・Review Billing Details
・Streamline R-SDV Tasks
For CRCs:
・Streamline Consent and Visit Management
・Automate Project Reporting
・Reduce and Centralize Workload
・Automate Cost Aggregation
Partner Sites:
・・Manage candidate referrals from satellites
・・Sharing of progress information to satellites
Sub-CRCs:
・Task Delegation
・Visit-Specific Task Management
・Support During Main CRC Absences
Clinical Research Centers:
・Real-Time Project Status
・Instant Reporting from CRCs
・Milestone Billing Management
・Expense Management
・Automated Revenue Forecasting
Clinical Trial-Related Departments:
・Simplify request submissions for lab tests
・Efficiently manage test schedules with task tools
