Dr. Yasuo Takeda, Chairman of the Japanese Society of Hospital Pharmacists, has been appointed as an advisor to our company.
Press
[Webinar] DCT model created by medical institutions in collaboration with partner sites

Buzzreach will be hosting a webinar titled “StudyWorks’ New Role in Supporting Medical Institution-Led DCT.” We will discuss the latest trends in decentralized clinical trials and new features of StudyWorks® that can be used in the field. We will also touch on the new StudyWorks feature, DCT business management, which
was released in a press release on Wednesday, September 10th . We hope that this will be helpful for those involved in DCT, especially those conducting clinical trials, in promoting their work.
◆ Event details
Date and time: Wednesday, October 8, 2025, 3:00 PM to 4:00 PM (doors open from 2:45 PM)
Format: Online (Zoom live streaming)
Participation fee: Free (pre-registration required / maximum 500 people)
Registration deadline: Wednesday, October 8, 2025, 1:00 PM
Target audience: Those involved in clinical trial work at clinical trial medical institutions and related stakeholders
Application URL: https://us06web.zoom.us/webinar/register/WN_AsR7OvQRSTuZwg85SG1JZA

Part 1: Rethinking the position of the Japanese version of DCT ~
The near future of the clinical trial industry as digital transformation advances~
Speaker: Shuji Fukunaga, General Manager, Site Relations Department, Platform Promotion Headquarters, Buzzreach

Part 2: Buzzreach’s support for clinical trial sites x partner site DCT initiatives
– DCT business support using StudyWorks –
Lecturer: Kazuya Inoue, Senior Manager, BD Team, Site Relations Department, Platform Promotion Headquarters, Buzzreach
| ■ Background of the new features |
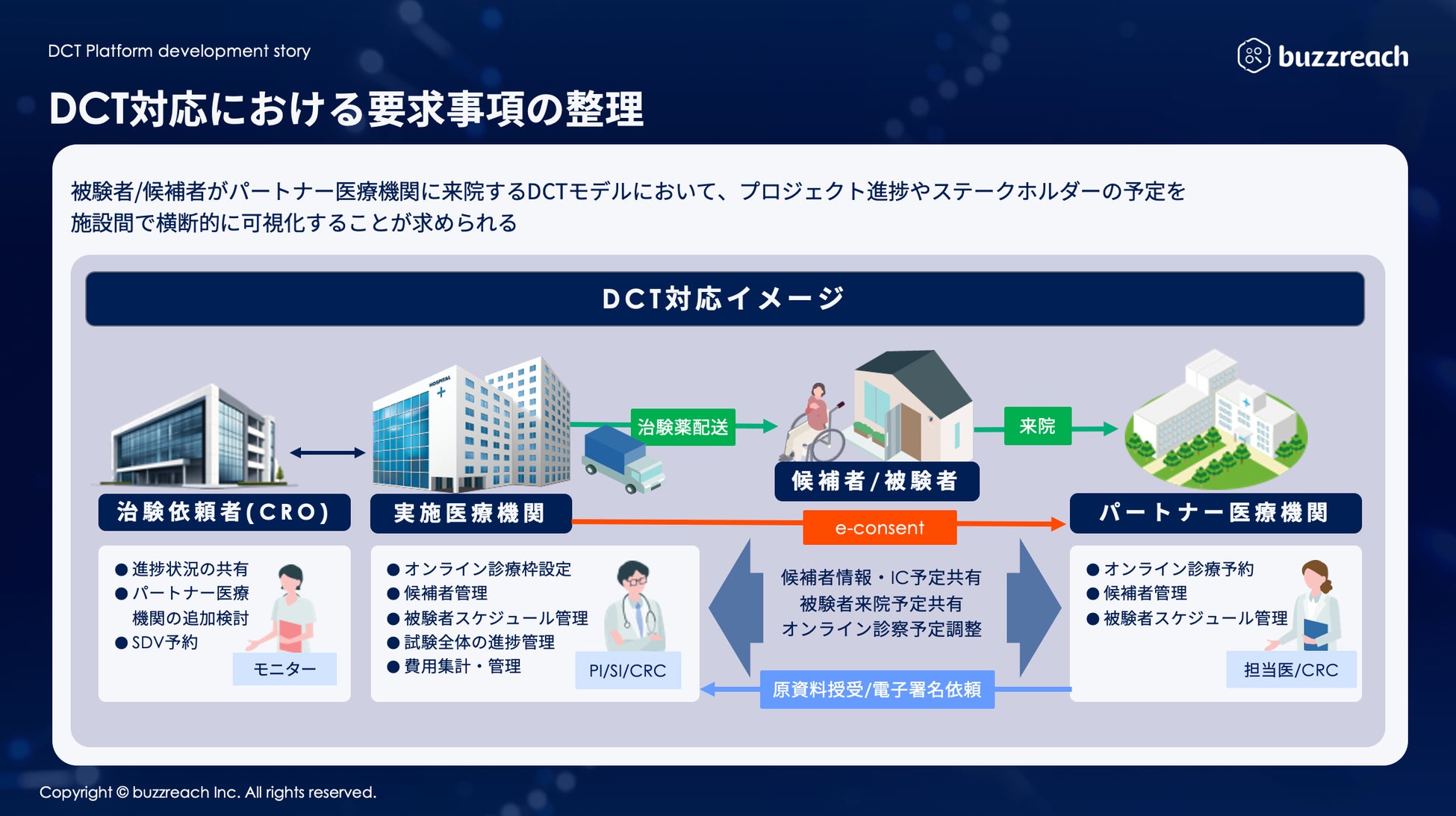
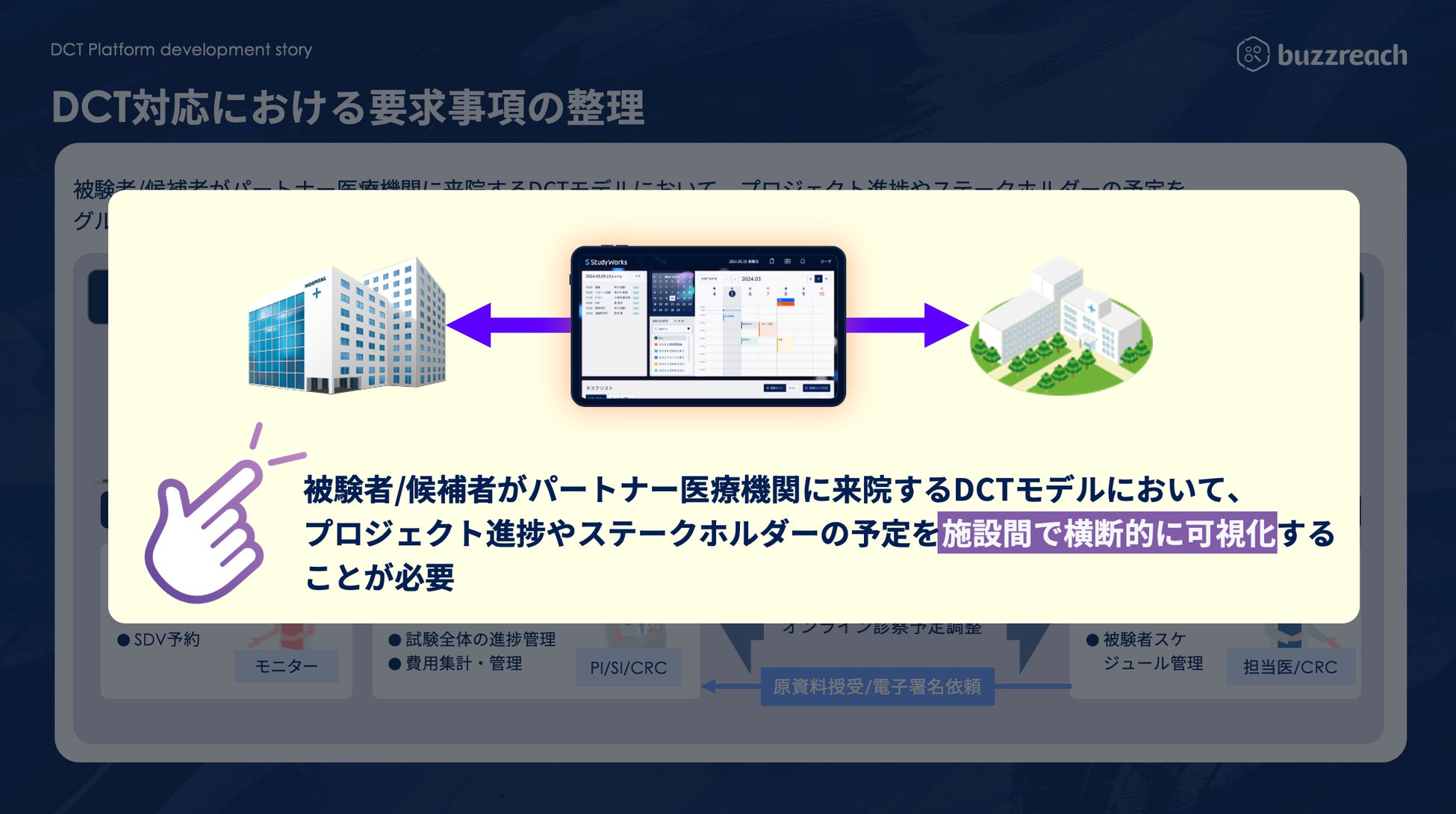
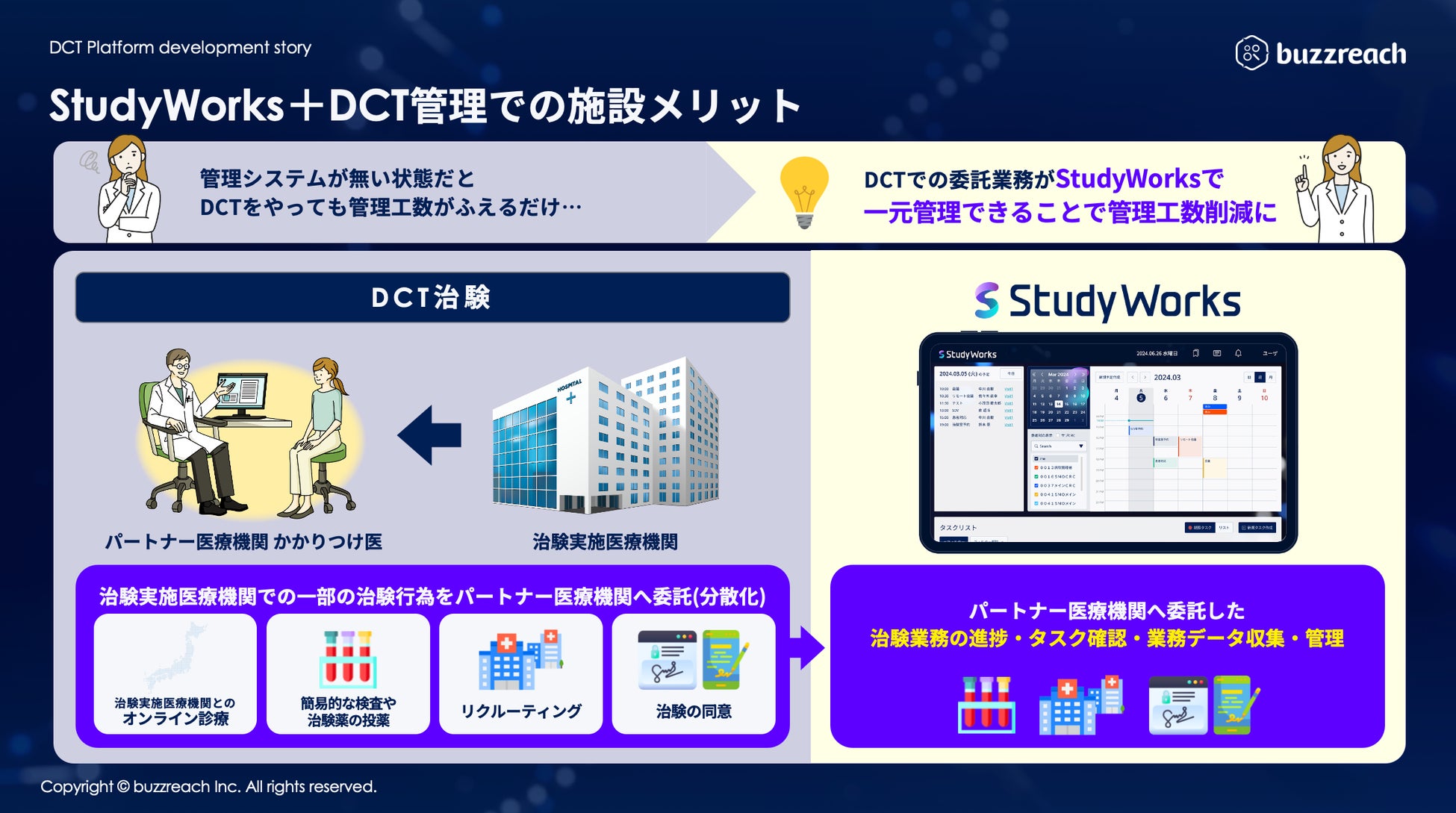
As the government develops DCT promotion policies and related guidelines, the scope of clinical trial operations that can utilize partner sites has been clarified and is expanding in practice. A wide range of operations, from recruiting support (e-recruitment) to obtaining subject consent (e-consent) and medical support (visit support, online medical consultations, visiting nurses, etc.), are rapidly expanding within the framework of decentralized clinical trials. At the same time, it is essential for clinical trial medical institutions to accurately understand the content of outsourced operations and manage them appropriately . Until now, they have been forced to manage each DCT operation separately, which has resulted in situations where the burden on sites has increased, and concerns have been raised that this goes against the philosophy of promoting DCT.
To address these challenges, Buzzreach will begin offering the first DCT task management function in StudyWorks®, creating an environment where medical institutions can centrally manage complex DCT tasks.
| ■ StudyWorks® × DCT business management function support |
As of August 12, 2025, the following DCT-specific features have been added to StudyWorks®.
・Partner site patient information management:
Online, centralized management of subjects admitted via partner sites.
・Partner site progress dashboard:
Real-time understanding of clinical trial progress at each partner site.
・Online medical appointment management:
Streamline consultation schedule coordination and reservations with principal investigators and sub-physicians.
・Visit scheduling:
Centralized management of visit and hospital visit schedules at partner sites on the platform.
・Contract task management:
Accurately track the status of tasks outsourced to partner sites.
・Automatic cost calculation function:
Automatically calculates DCT-related costs such as visit fees and patient copayment reduction fees, streamlining billing operations.
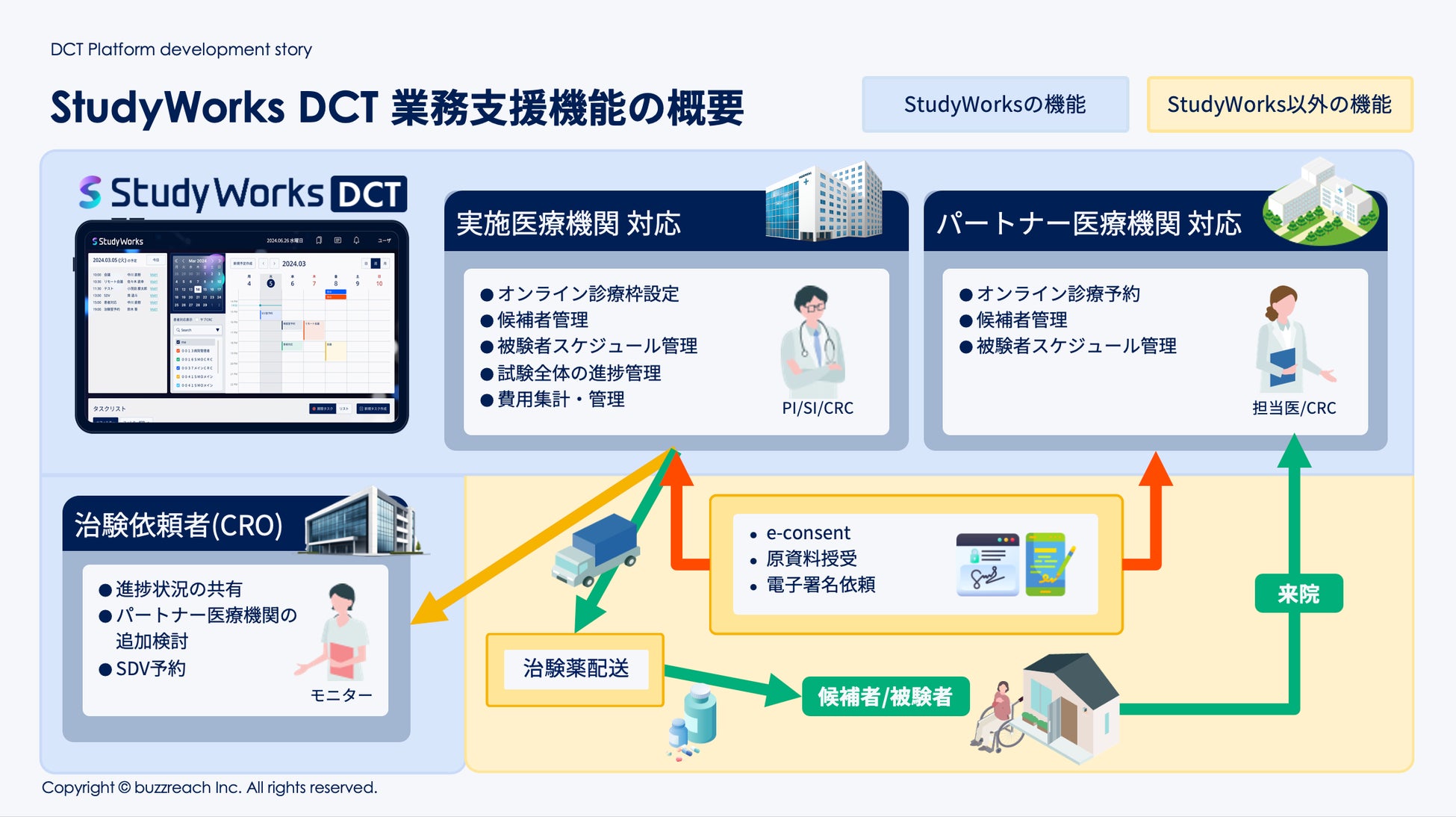
In order to smoothly carry out clinical trial-related work with partner sites, we have established a digital infrastructure that enables medical institutions, CROs, and pharmaceutical companies to smoothly operate DCT.
Buzzreach will continue to support the acceleration of clinical trials and the optimization of operations through StudyWorks® and its partner site collaboration model.