To promote clinical trial digital transformation, we have entered into a capital and business alliance with Medley M3 and raised funds from DG Resona.
Press
We have begun offering the first DCT business management function on our clinical trial and clinical research business support platform, StudyWorks®.

– Promoting DCT efficiency through support for partner sites, online medical appointments, and automatic cost calculations –
Buzzreach Inc. (Head office: Minato-ku, Tokyo; Representative Director and CEO: Takateru Inokawa) has enhanced the management functions for DCT*1 (Decentralized Clinical Trial) operations in its clinical trial and clinical research support platform ” StudyWorks®︎ ,” and began offering it on August 12, 2025. It has already begun to be used in clinical trials currently being conducted by Tokushukai General Incorporated Association (Location: Chiyoda-ku, Tokyo), and provides a comprehensive solution to handle increasingly complex clinical trial operations. Click
here for the press release .
■ Background of the new features
As the government develops DCT promotion policies and related guidelines, the scope of clinical trial operations that can utilize partner sites has been clarified and is expanding in practice. A wide range of operations, from recruiting support (e-recruitment) to obtaining subject consent (e-consent) and medical support (visit support, online medical consultations, visiting nurses, etc.), are rapidly expanding within the framework of decentralized clinical trials. At the same time, it is essential for clinical trial medical institutions to accurately understand the content of outsourced operations and manage them appropriately . Until now, they have been forced to manage each DCT operation separately, which has resulted in situations where the burden on sites has increased, and concerns have been raised that this goes against the philosophy of promoting DCT.
To address these challenges, Buzzreach will begin offering the first DCT task management function in StudyWorks®, creating an environment where medical institutions can centrally manage complex DCT tasks.
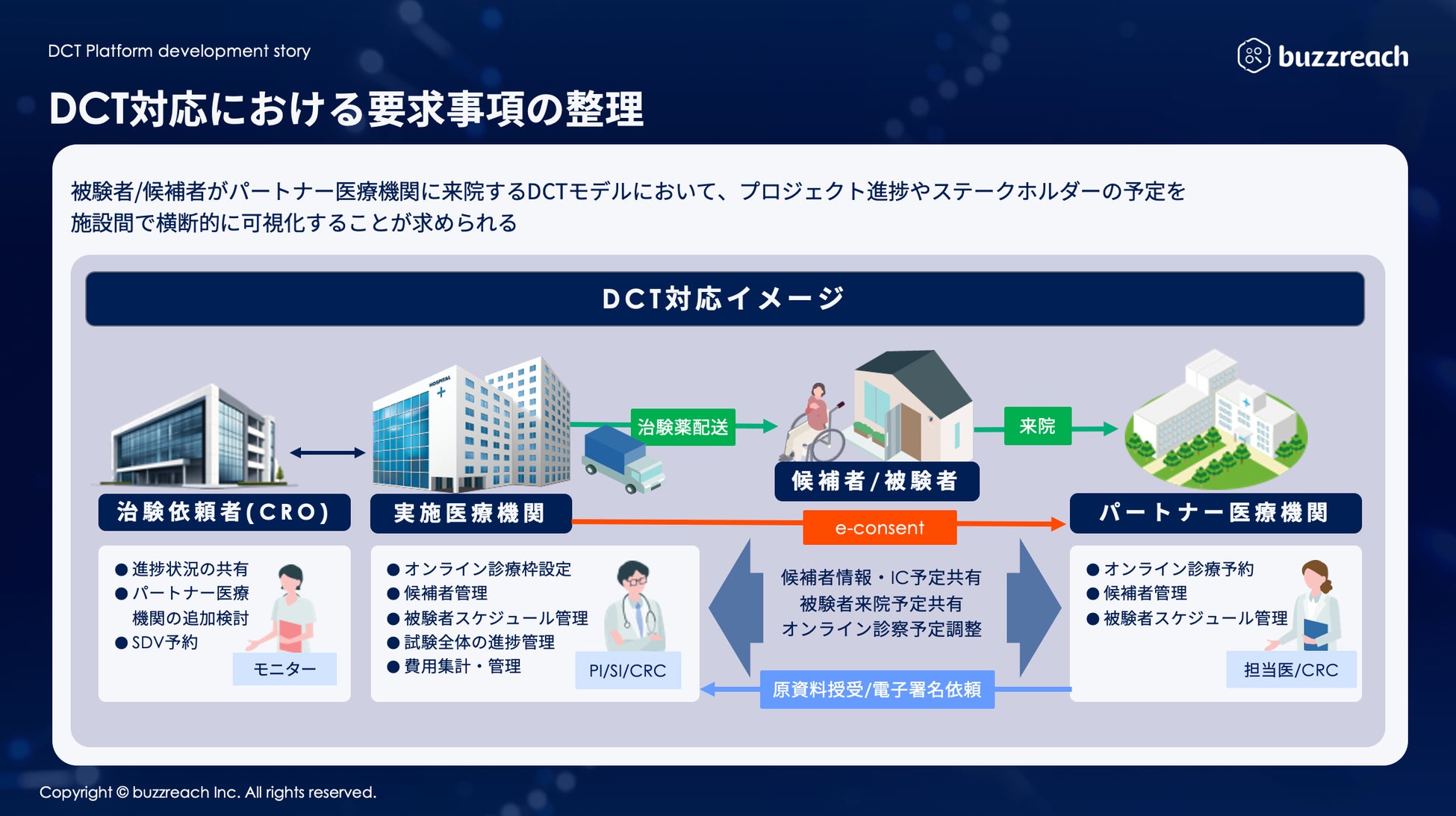
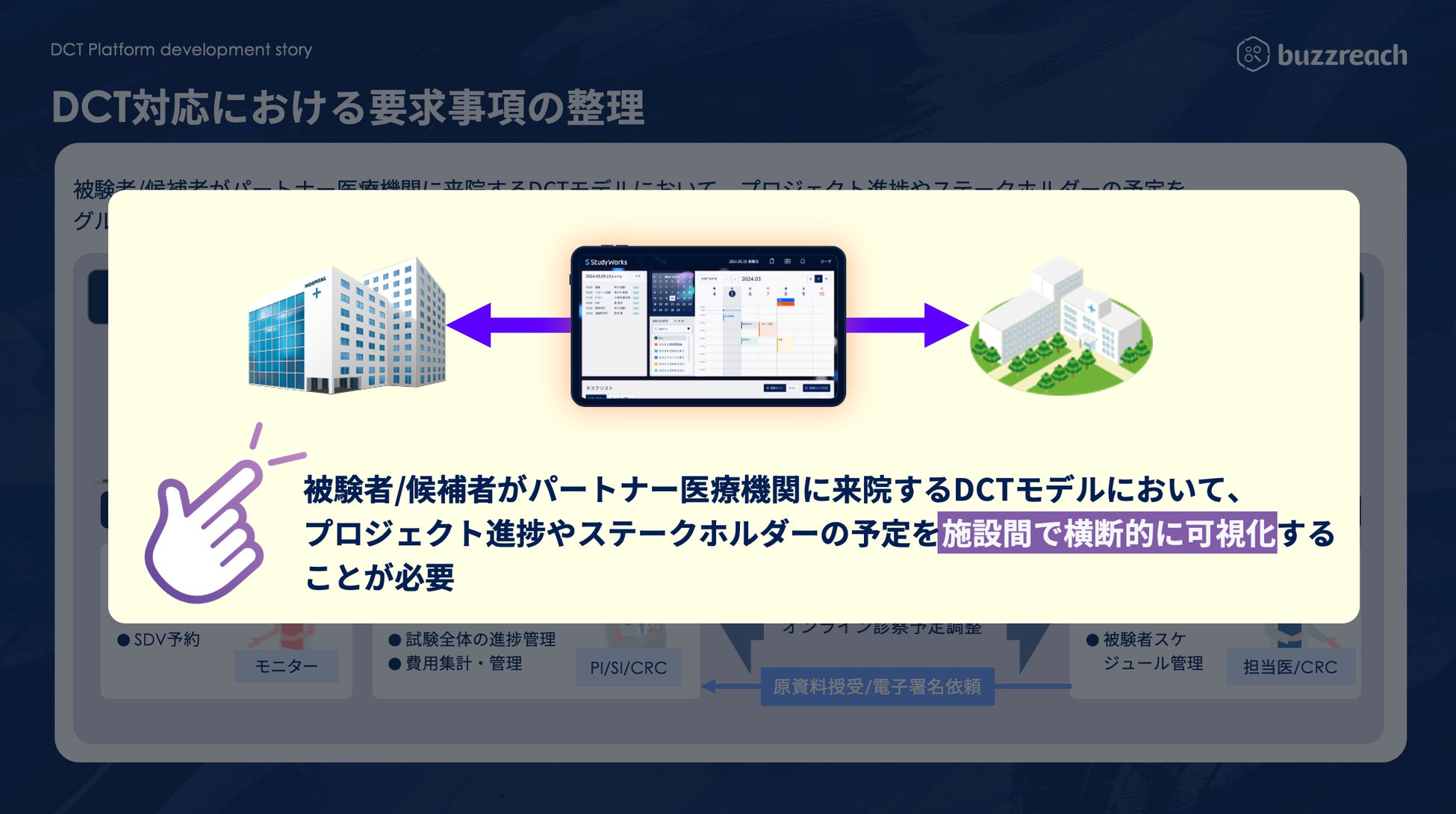
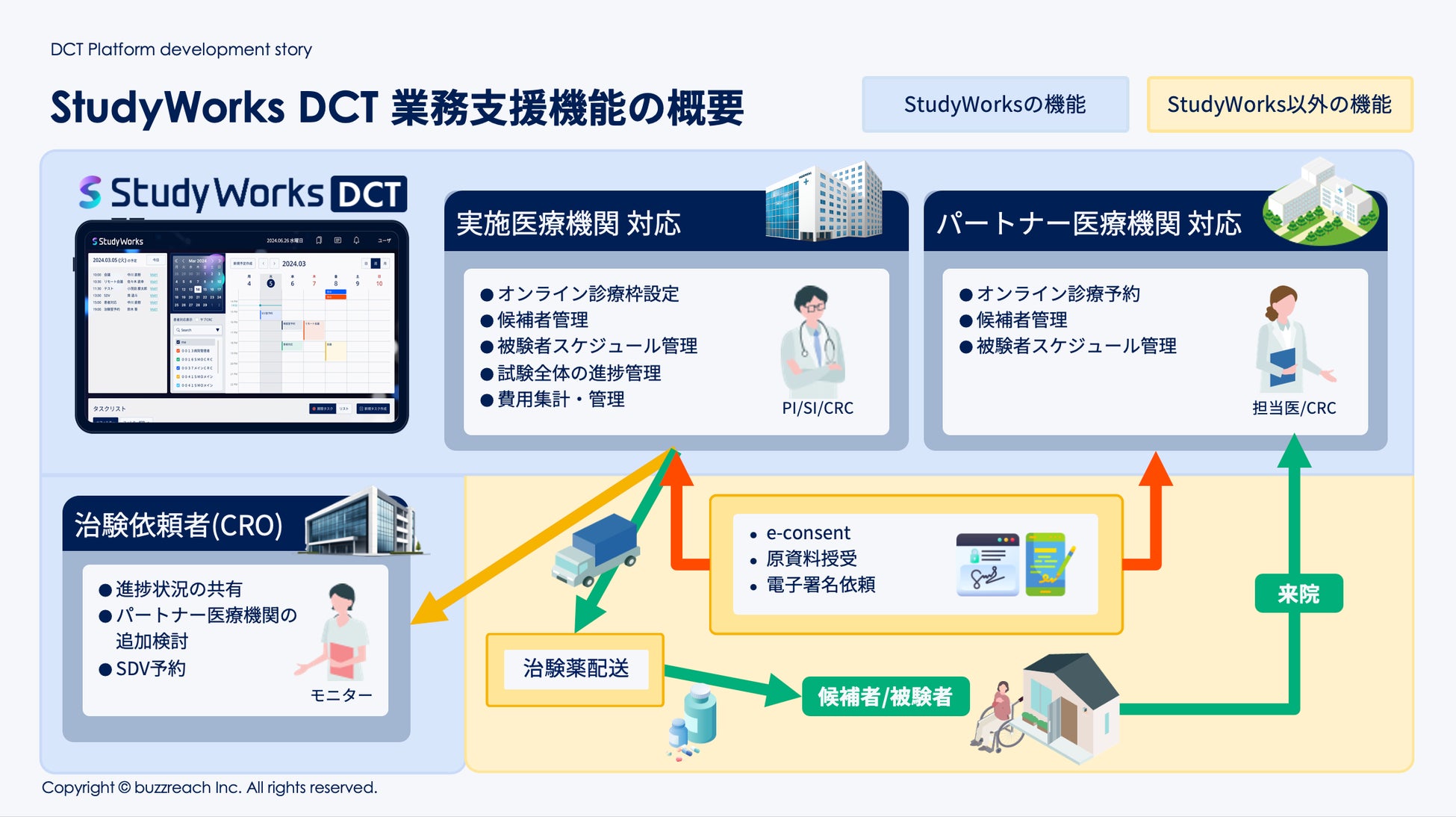
■ StudyWorks® × DCT business management function support
As of August 12, 2025, the following DCT-specific features have been added to StudyWorks®.
・Partner site patient information management:
Online, centralized management of subjects admitted via partner sites.
・Partner site progress dashboard:
Real-time understanding of clinical trial progress at each partner site.
・Online medical appointment management:
Streamline consultation schedule coordination and reservations with principal investigators and sub-physicians.
・Visit scheduling:
Centralized management of visit and hospital visit schedules at partner sites on the platform.
・Contract task management:
Accurately track the status of tasks outsourced to partner sites.
・Automatic cost calculation function:
Automatically calculates DCT-related costs such as visit fees and patient copayment reduction fees, streamlining billing operations.
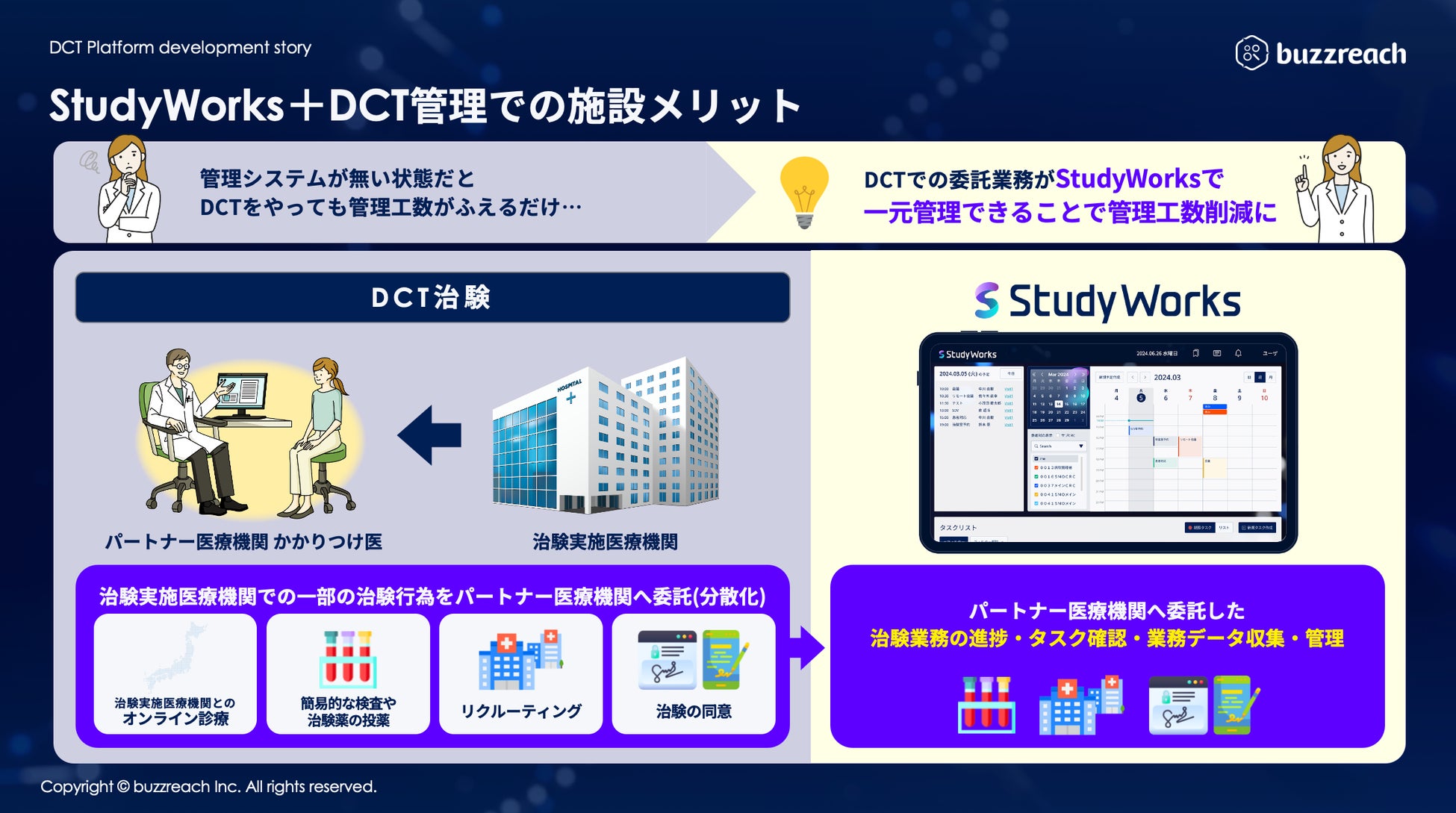
In order to smoothly carry out clinical trial-related work with partner sites, we have established a digital infrastructure that enables medical institutions, CROs, and pharmaceutical companies to smoothly operate DCT.
Buzzreach will continue to support the acceleration of clinical trials and the optimization of operations through StudyWorks® and its partner site collaboration model.
■ Comments from Tokushukai Group representatives
When Tokushukai Group decided to conduct a DCT clinical trial, we encountered tasks that were different from those in traditional clinical trials, such as collaboration between the implementing medical institution and partner medical institutions, sharing candidate information, and adjusting subject schedules, and we were very worried about how to handle these tasks. By utilizing the DCT function of StudyWorks®, we can manage subject progress, online medical appointments, cost aggregation, and more on a common platform. Another strength of StudyWorks® is that it can collaborate not only with the implementing medical institution and partner medical institutions, but also with the clinical trial sponsor and CRAs through this platform, which we believe will directly reduce the burden on the field and speed up the clinical trial.
■ Information about related webinars

In connection with this release, Buzzreach will be hosting a webinar titled “StudyWorks: A New Role for Supporting Medical Institution-Led DCT.”
The webinar will discuss the latest trends in decentralized clinical trials and new features of StudyWorks® that can be used in the field. We hope that it will be useful for promoting the work of everyone involved in DCT, particularly clinical trial sites.
◆ Event details
Date and time: Wednesday, October 8, 2025, 3:00 PM – 4:00 PM (doors open from 2:45 PM)
Format: Online (Zoom live broadcast)
Participation fee: Free (pre-registration required / maximum 500 people)
Registration deadline: Wednesday, October 8, 2025, 1:00 PM
Application URL: https://us06web.zoom.us/webinar/register/WN_AsR7OvQRSTuZwg85SG1JZA

Part 1: Rethinking the position of the Japanese version of DCT ~
The near future of the clinical trial industry as digital transformation advances~
Speaker: Shuji Fukunaga, General Manager, Site Relations Department, Platform Promotion Headquarters, Buzzreach

Part 2: Buzzreach’s support for clinical trial sites x partner site DCT initiatives
– DCT business support using StudyWorks –
Lecturer: Kazuya Inoue, Senior Manager, BD Team, Site Relations Department, Platform Promotion Headquarters, Buzzreach
■About “StudyWorks®︎”
“StudyWorks®” is a business efficiency platform for clinical trial medical institutions, and has the following features:
Centralized management of clinical trial work: Manage various clinical trial-related tasks on a single platform, making progress visualization and task management easy. Smooth communication between parties involved: All stakeholders involved in clinical trials, including CRCs (clinical trial coordinators), doctors, and pharmaceutical companies (including CROs), can share information in real time, improving communication efficiency. Strengthened quality control: Maintain and improve the quality of clinical trial work through standardized processes and checklists. Cost aggregation function: Automatically aggregates clinical trial expenses such as subject burden reduction fees and research costs, allowing you to check the occurrence date and breakdown at a glance. This makes it easier to check billing details and is expected to improve the efficiency of billing work. It also complies with benchmark-type costs associated with Fair Market Value (FMV), which is expected to be introduced in the future. StudyWorks service site

■ Future outlook
Buzzreach will continue to deploy “StudyWorks®” to clinical trial medical institutions nationwide, contributing to the elimination of drug lag/loss issues in Japan and the improvement of new drug development capabilities, thereby contributing to the efficiency and quality of clinical trial operations. In addition
to the issues raised in the recent Ministry of Health, Labor and Welfare-led clinical trial ecosystem*1, including (1) issues that significantly contribute to cost reduction,
(2) issues that significantly contribute to improving case accumulation, and (
3) issues that significantly contribute to improving clinical trial efficiency and shortening the time to start
, Buzzreach also applies Fair Market Value (FMV), a calculation method based on workload and market price, to optimize clinical trial costs promoted by authorities and industry groups. In particular
, with regard to issue (2) that significantly contributes to improving case accumulation , Buzzreach will support the creation of a new clinical trial network that utilizes partner sites that regularly cooperate with medical institutions in the surrounding area in collecting cases, starting from the clinical trial medical institution.
We will continue to strive to further improve our functionality and services based on feedback from our users. We will work with medical institutions, pharmaceutical companies, CROs, and government agencies to promote clinical trial digital transformation in Japan and build an ecosystem to popularize DCT (decentralized clinical trials). Through StudyWorks, we will communicate its practical value for the present and future of clinical trials, helping to accelerate new drug approvals.
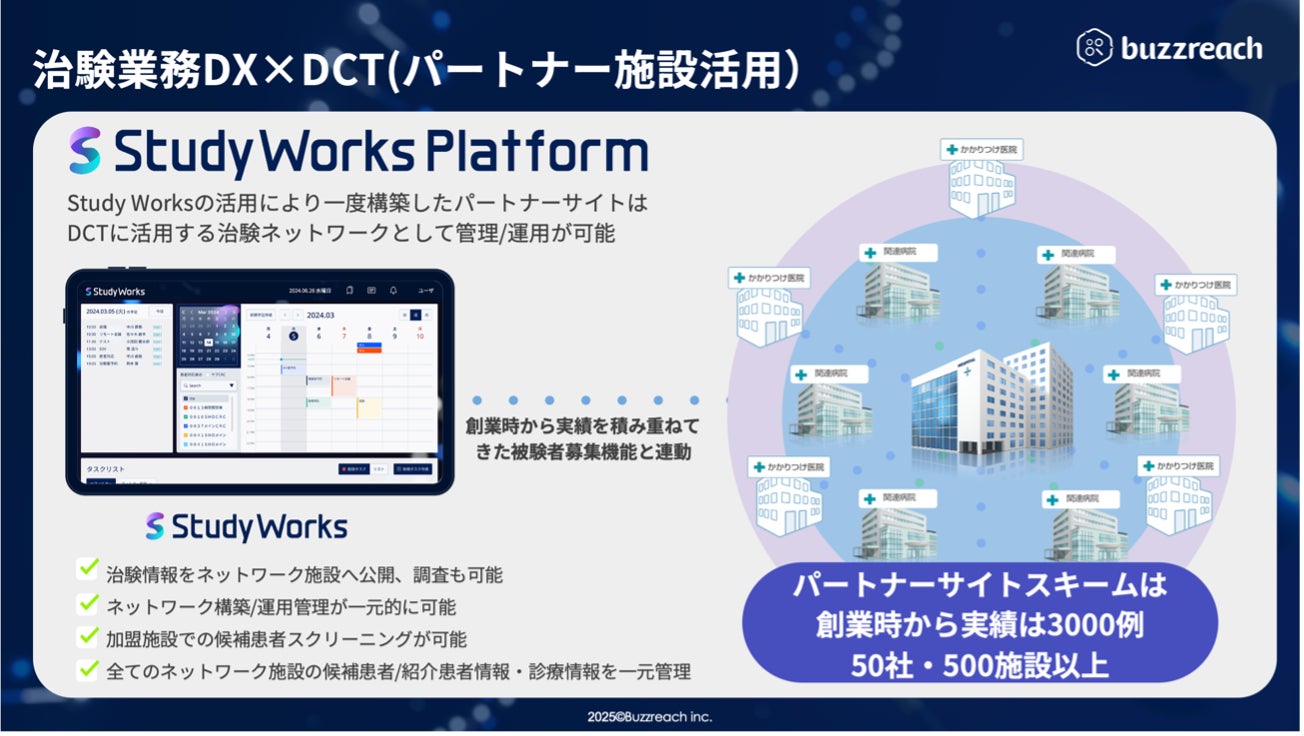
Utilizing permanent partner sites that support case accumulation linked to StudyWorks
*1 DCT: Decentralized Clinical Trial is a new method for expanding clinical trial options. It is an initiative to improve the efficiency of clinical trials by working with partner sites, which are cooperating medical institutions, rather than consolidating all clinical trial operations at the medical institution conducting the clinical trial.
*2 Clinical trial ecosystem: A system led by the Ministry of Health, Labor and Welfare in which various stakeholders, including pharmaceutical companies, medical institutions, regulatory authorities, and subjects, cooperate to efficiently conduct clinical trials in order to quickly deliver therapeutic drugs to the public.
[Contact for inquiries regarding this matter]
Buzzreach Inc. Public Relations
Email: info@buzzreach.co.jp
URL: https://www.buzzreach.co.jp/contact
Company Name: Buzzreach
Inc. Head Office: Minato-ku, Tokyo
CEO: Takateru Inokawa
Business Description: Provision of clinical trial and clinical research support SaaS, DCT support business, development and operation of patient recruitment platform utilizing partner sites, patient SNS business, etc.
URL: https://www.buzzreach.co.jp/






