To promote clinical trial digital transformation, we have entered into a capital and business alliance with Medley M3 and raised funds from DG Resona.
Press
“StudyWorks” now installed at over 30 facilities, and now also supports DCT business management

“StudyWorks,” which promotes the digitalization of clinical trial operations, has been adopted by over 30 facilities, and now also supports DCT operation management
— Adoption is accelerating, primarily at university hospitals and group hospitals, fueled by the trend toward digital transformation in clinical trials —
Buzzreach Inc. (Head office: Minato-ku, Tokyo; CEO: Takateru Inokawa) is pleased to announce that its clinical trial support SaaS, “StudyWorks,” has been introduced to approximately 30 designated treatment hospitals and core hospitals nationwide, which conduct numerous clinical trials, as of May 2025. The company also announces that it will begin offering a portion of its “DCT business management functions, including partner sites*,” which are compatible with DCT (decentralized clinical trials*), starting this summer.
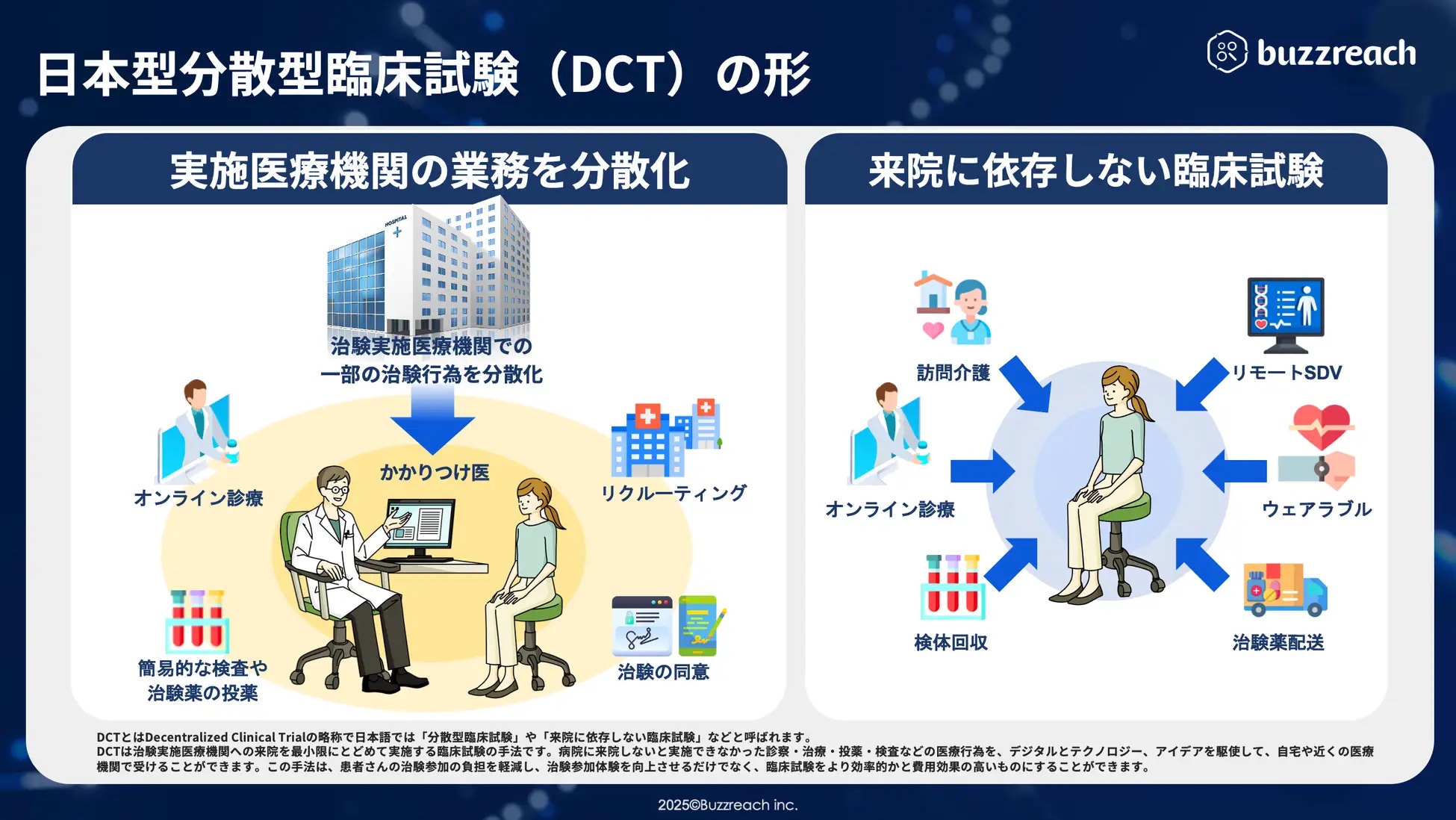
*DCT (Decentralized Clinical Trial) is a new method for expanding clinical trial options. It is an initiative to improve the efficiency of clinical trials by working with cooperating medical institutions called partner sites, rather than concentrating all clinical trial operations at the medical institution conducting the clinical trial.

■ Introduction is expanding to designated functioning hospitals and core hospitals , primarily university hospitals
“StudyWorks” is a SaaS-based tool that supports clinical trial and clinical research operations. It was officially launched in 2024, and as of May 2025, it has been introduced at designated treatment hospitals and core hospitals that conduct multiple clinical trials and clinical research simultaneously, such as Tohoku University Hospital, Kanazawa University Hospital, Tokushukai Group, Saiseikai Group, National Center for Global Health and Medicine, National Institute for Health Risk Management, Tokyo Medical University Hospital, JA Ibaraki Prefecture Health Federation Tsuchiura Kyodo Hospital, and National Hospital Organization Chiba Higashi Hospital, bringing the total number of facilities that have adopted the tool to over 30.
■ Main reasons for implementation: FMV* compliant cost management, standardization of CRC work, complex schedule management
Medical institutions that have adopted the system have given it high praise for the following points:
At large hospitals that conduct multiple clinical trials simultaneously, these functions will greatly contribute to strengthening the clinical trial implementation system, such as by supporting a clinical trial cost calculation process based on FMV (Fair Market Value), reducing administrative burdens with an automatic calculation function for clinical trial costs, centralizing the work of CRCs (clinical trial coordinators) centered on managing the complex schedules of patients participating in clinical trials, and standardizing clinical trial work and visualizing progress
. *FMV (Fair Market Value) refers to clinical trial costs that are calculated after the medical institution and clinical trial sponsor (pharmaceutical company, etc.) understand the clinical trial protocol, are agreed upon by both parties, and are deemed reasonable by a third party, with the aim of setting fair and transparent compensation for the various tasks and resources involved in clinical trials.
■ Full-scale start of DCT business management support
Starting around summer 2025, StudyWorks will expand its DCT (Distributed Clinical Trials) business management capabilities. As a result of deregulation of GCP*, the number of clinical trial tasks that can be outsourced to partner sites has increased, starting with recruiting support (e-recruitment), and including clinical trial consent (e-consent) and clinical trial medical support (visit support, online medical consultations, visiting nurses, etc.) in line with the government’s promotion of DCT. As such, clinical trial medical institutions need to understand the details of these outsourced tasks. Until now, each DCT task had to be managed separately, which increased the burden on clinical trial medical institutions, raising concerns about an environment that was the exact opposite of promoting DCT.
To overcome these challenges, we will begin providing some DCT business management functions
. *GCP: Good Clinical Practice (GCP) is a standard established by the Ministry of Health, Labor and Welfare regarding the implementation of clinical trials for pharmaceuticals.
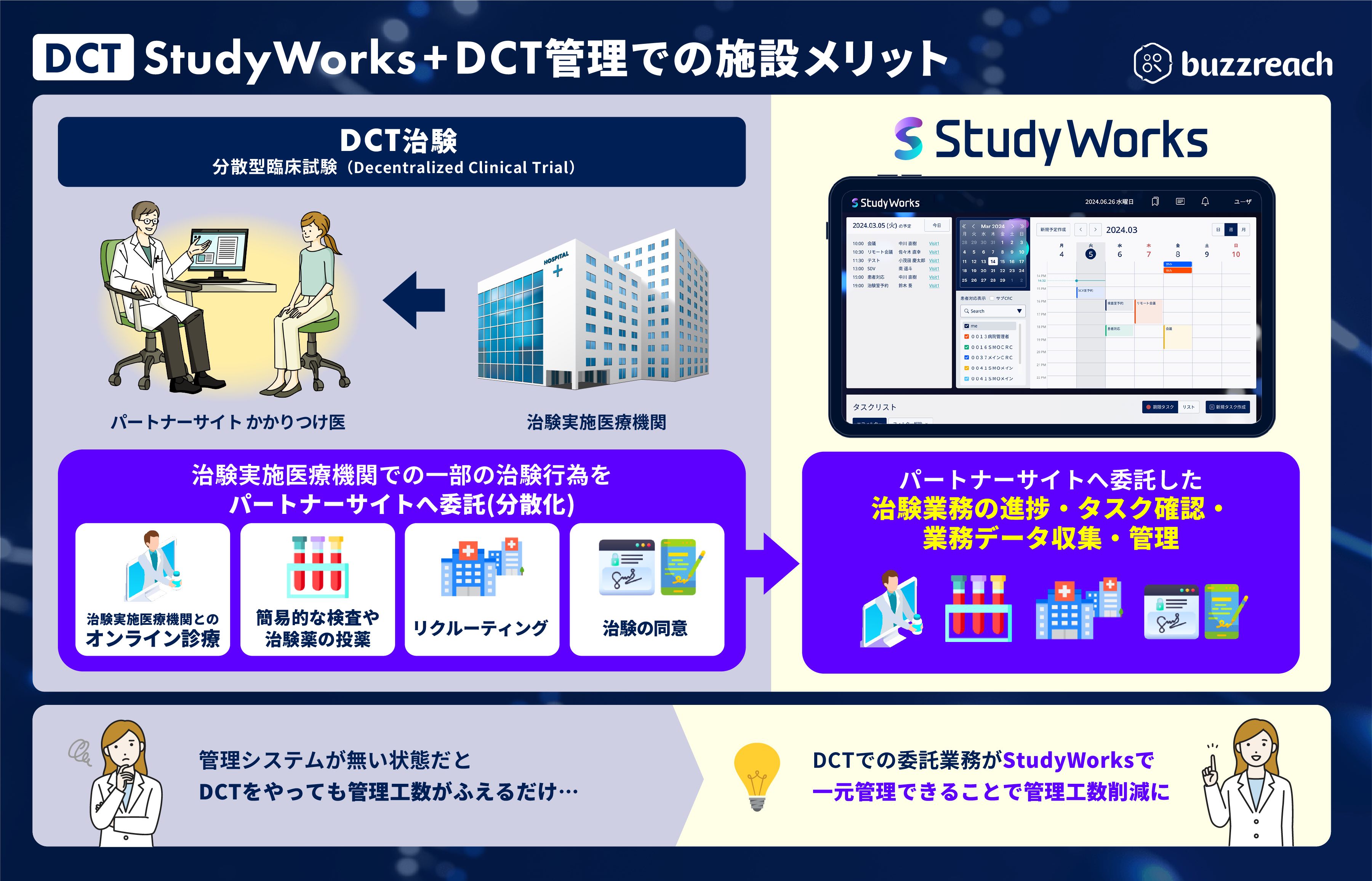
《Functions to be provided》
■ Online screening and patient information management associated with patient recruitment at partner sites
■Progress management for each partner site
■Reservation management for online consultations, etc. to ensure that clinical investigators have time slots for treatment
■Visit schedule management handled by partner sites
■Task management for outsourced clinical trial work
■Cost aggregation for outsourced clinical trial-related work, etc.
In order to smoothly carry out clinical trial-related work with partner sites, we will develop a digital infrastructure that will enable medical institutions, CROs, and pharmaceutical companies to smoothly operate DCT.
Buzzreach will continue to support the acceleration of clinical trials and the optimization of operations through “StudyWorks” and the partner site collaboration model.
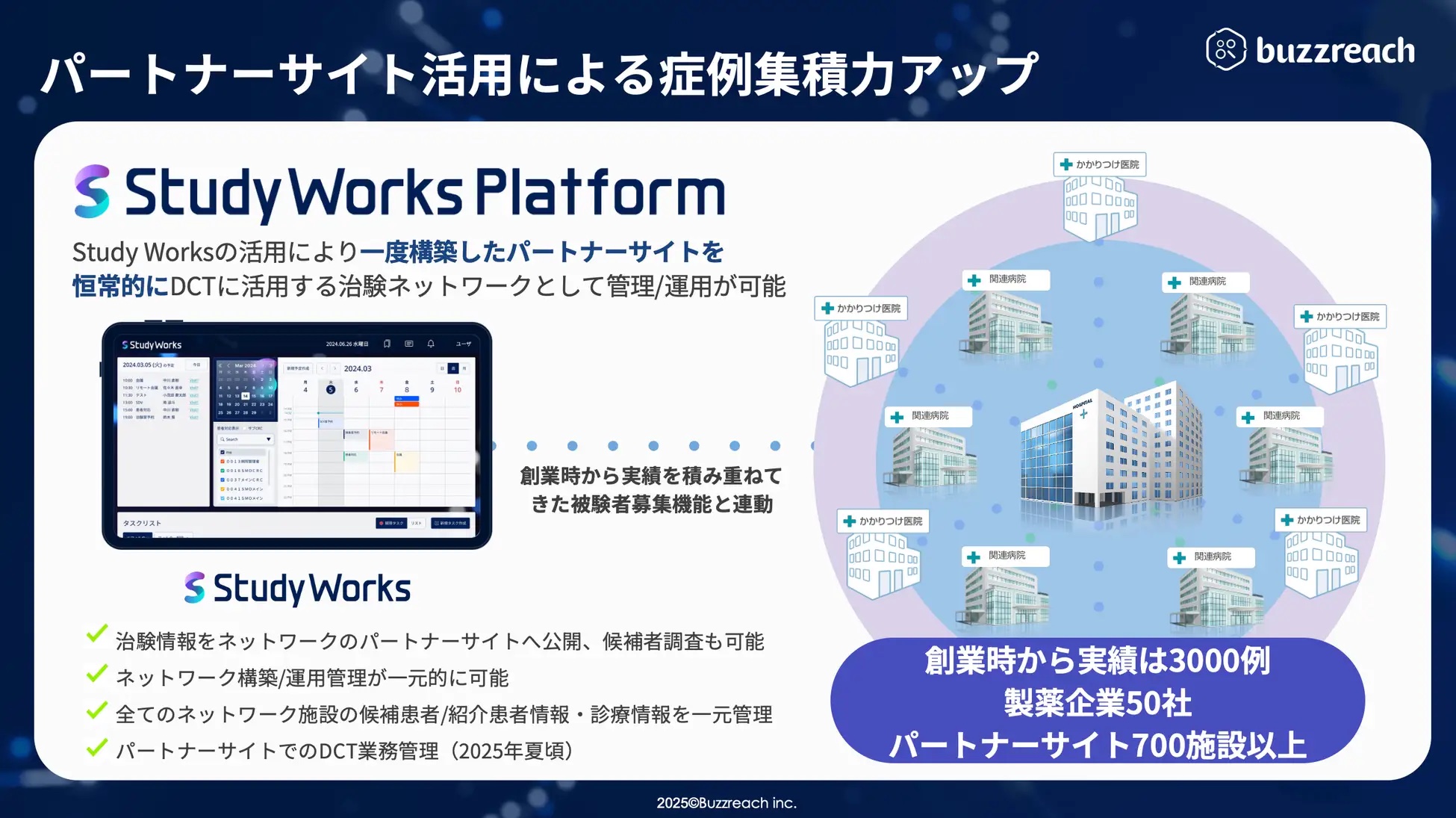
About StudyWorks
StudyWorks is a SaaS-based business support tool that streamlines clinical trial and clinical research operations. It provides all the functions required for clinical trial management, such as automatic calculation of clinical trial costs that also supports FMV, CRC work management, and progress reports, all in one place. From the summer of 2025, it will also begin supporting outsourced work management to partner sites in the promotion of DCT, and is expected to be used as a digital transformation platform for clinical trial operations at medical institutions in the future.
https://product.puzz.app/lp/sw/
Click here for the PR Times press release






