We have begun offering the first DCT business management function on our clinical trial and clinical research business support platform, StudyWorks®.
Press
To promote clinical trial digital transformation, we have entered into a capital and business alliance with Medley M3 and raised funds from DG Resona.

| Supporting case accumulation across each company’s assets in the medical healthcare field and strengthening the creation of a Japanese version of the DCT ecosystem |
Our company aims to solve the challenges of new drug development by developing a platform specialized in the clinical trial and clinical research fields, focusing on clinical trial operations DX SaaS ” Study Works® ” and patient recruitment support. We have entered into a capital and business alliance agreement with Medley Inc. (Head office: Minato-ku, Tokyo; President and CEO: Takiguchi Kohei; hereinafter “Medley”) and M3, Inc. (Head office: Minato-ku, Tokyo; Representative Director: Tanimura Itaru; hereinafter “M3”), and have also raised funds from DG Resona Ventures Investment Limited Partnership No. 1 (Location: Shibuya-ku, Tokyo; hereinafter “DG Resona”) . Through this alliance and funding, we will utilize the medical, healthcare, and financial assets of each company to improve case accumulation rates and clinical trial implementation rates, and strengthen the social implementation of a Japanese version of the DCT (decentralized clinical trial) ecosystem. Click
here for the press release .
■ Background and purpose of capital and business alliance and fundraising
Drug lag and drug loss caused by delays in new drug development and an increase in unapproved drugs are major challenges in building a stable medical system in Japan.In addition, in the area of clinical trials and research, there are several issues that need to be resolved in order to speed up development and research, such as “information about new drugs is not reaching the patients and medical institutions who need it” and “digitalization of clinical trial work is not progressing.”
To solve these issues, the government has launched a series of measures to stimulate new drug development, such as deregulation, the creation of a clinical trial ecosystem*1, and the promotion of DCT*2, and has begun various initiatives to enable medical institutions conducting clinical trials to be involved in more clinical trials and research more quickly.In
particular, with regard to DCT, the government is supporting the development of a digital transformation environment necessary to make clinical trials a more familiar medical option, through measures such as e-recruitment, which recruits patients to participate in clinical trials online, e-consent using electronic consent tools, online medical consultations that allow clinical trial treatment to be received at home or by a primary care physician, medical support through visiting nurses at patients’ homes, and relaxation of delivery restrictions for investigational drugs to patients’ homes, pharmacies, and primary care physicians.
In this environment, Buzzreach offers multiple services, including the clinical trial and research support platform “StudyWorks,” the clinical trial information disclosure and management function “smt,” case collection support for DCT-type subject recruitment using partner sites, and the patient-specific SNS service “Miraiku,” to enable centralized management of clinical trial operations on a platform targeted at university hospitals, cancer centers, large group hospitals, etc., as well as centralized management of partner site*3 outsourced operations that are compatible with the Japanese-style DCT.
Through this capital alliance and fundraising with these companies, we will not only take steps to solve new challenges in the field of clinical trials and clinical research, but also work to provide new value to the medical institutions and patients who use the services of these companies.
*1 A system led by the Ministry of Health, Labor and Welfare to efficiently conduct clinical trials through cooperation between pharmaceutical companies, medical institutions, regulatory authorities, and patient subjects.
*2 Decentralized Clinical Trials. Conventionally, clinical trial-related tasks such as patient recruitment, in-patient consultations, and medication all had to be carried out at the medical institution conducting the clinical trial. However, by utilizing e-recruitment, e-consent, online consultations, visiting nurses, and investigational drug delivery, this method allows patients to participate in clinical trials from locations other than medical institutions, such as their family doctor or at home.
*3 Clinical trial collaborating medical institutions associated with DCT
■ Future role of Buzzreach
Through this partnership, Buzzreach will focus on promoting the following:
– Standardization and automation of clinical trial work digital transformation based on “Study Works”
– Strengthening the case accumulation capacity of clinical trial medical institutions by expanding the partner site network
– Social implementation of a Japanese-style DCT that combines the assets of each company
– Establishment of a system for issuing investigational drugs and follow-up, including in pharmacies and home care
– Concrete contribution to the government’s “Clinical Trial Ecosystem Initiative”
■ Comment from the CEO (Takaaki Inokawa, CEO of Buzzreach Inc.)

This capital alliance and fundraising will accelerate the social implementation of a Japanese version of DCT by strengthening case accumulation at clinical trial medical institutions through partner site-based patient recruitment and leveraging the assets of each company. Through this collaboration, we will collaborate with partners with different strengths: Medley, a leader in medical digital transformation; M3 and its group companies, a leading medical information service company with approximately 90% of Japan’s physicians registered; and DG Resona, which has a financial and payment infrastructure. Japan’s clinical trial digital transformation market is expected to expand rapidly in the future, driven by policy support and the digitalization of clinical trial sites. As a “platformer leading the social implementation of a Japanese version of DCT” in this growth field, we will promote the standardization of clinical trial infrastructure across industry, government, and academia through this alliance. Guided by our philosophy of “bringing new options to as many patients as possible through the power of technology,” we will continue to collaborate with a variety of partners to create a new clinical trial infrastructure that organically connects patients, medical institutions, and pharmaceutical companies.
■ Contribution to university hospitals and other institutions through this partnership
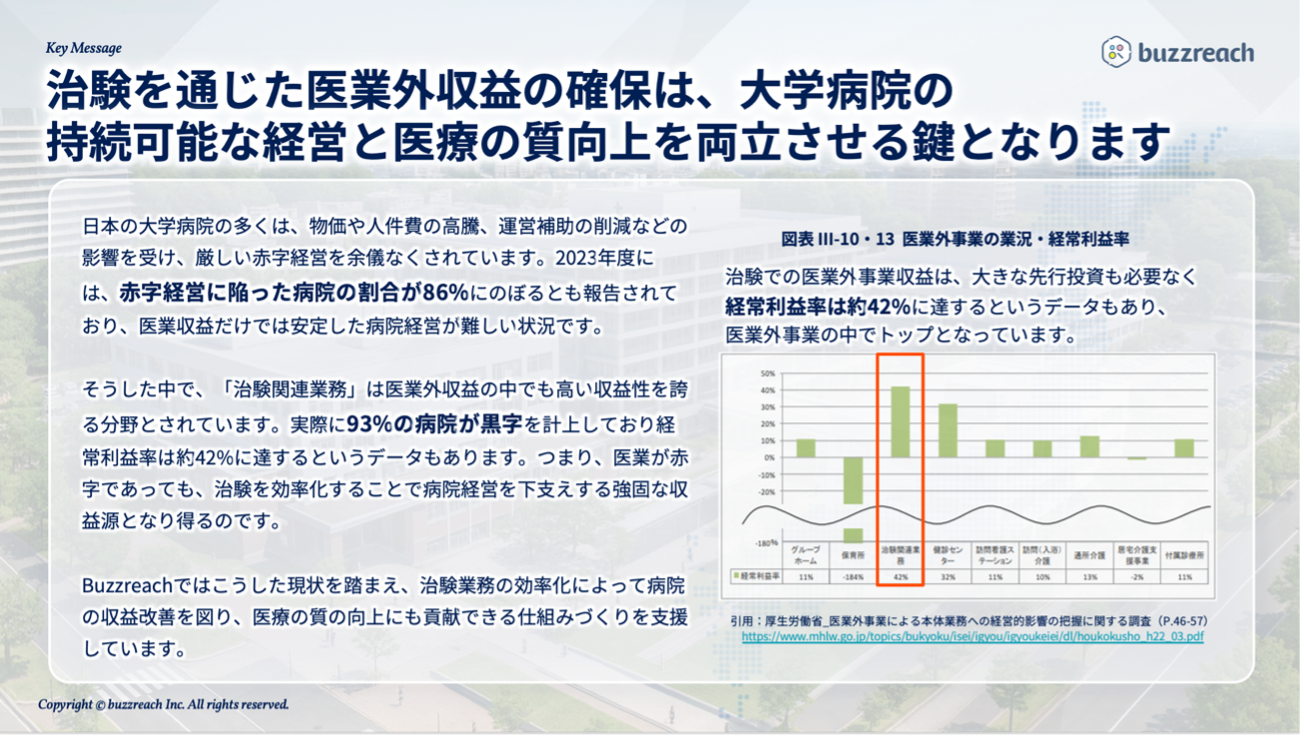
Many university hospitals, which play a central role in Japan’s clinical trial environment, are being forced to operate at a severe deficit due to the impact of rising prices and labor costs, as well as cuts in operating subsidies. It has been reported that by fiscal year 2023, the percentage of hospitals operating at a loss will reach 86%, making it difficult to maintain stable hospital management based solely on medical revenue. To help overcome this situation, this partnership will help develop clinical trial infrastructure that will lead to improved efficiency in clinical trial operations and support for increased case accumulation, thereby contributing to the development of university hospitals that conduct many clinical trials, the acceleration of clinical trials, and the implementation of medical innovation.
■ Company overview
Buzzreach Inc.
Address: Kowa Shirokanedai Building, 3-19-1 Shirokanedai, Minato-ku, Tokyo
Representative: Representative Director and CEO Takateru Inokawa
Business: Planning, development, and operation of clinical trial and clinical research support platforms
Main services: Provision of clinical trial and clinical research support SaaS, DCT support business, development and operation of patient recruitment platforms utilizing partner sites, patient SNS business, etc.
URL: https://www.buzzreach.co.jp
Medley Inc.
Location: Roppongi Hills Mori Tower 13F, 6-10-1 Roppongi, Minato-ku, Tokyo
Representative: President and CEO Kohei Takiguchi
Business: Medical platform business (CLINICS, MALL, DENTIS, MEDIXS, MINET, etc.), human resources platform business (JobMedley, JobMedley Academy, etc.)
URL: https://www.medley.jp
M3, Inc.
Address: Akasaka Intercity, 1-11-44 Akasaka, Minato-ku, Tokyo
Representative: CEO Itaru Tanimura
Business: Provision of medical-related services via the internet
URL: https://corporate.m3.com
DG Resona Ventures No. 1 Investment Business Limited Liability Partnership
Location: 15-1 Udagawacho, Shibuya-ku, Tokyo
Business: A CVC fund established to jointly promote open innovation and startup investment with the aim of creating business collaboration and synergies between Digital Garage (DG) and the Resona Group
URL: https://dg-resona-v.com/
■ Contact information regarding this matter
Buzzreach Inc. Public Relations
Email: info@buzzreach.co.jp
Website: https://www.buzzreach.co.jp/






