To promote clinical trial digital transformation, we have entered into a capital and business alliance with Medley M3 and raised funds from DG Resona.
Press
Using “smt,” Boehringer Ingelheim Japan begins supporting the disclosure of clinical trial information

Buzzreach Inc. (Headquarters: Minato-ku, Tokyo; CEO: Takateru Inokawa; hereinafter “Buzzreach”), which operates a clinical trial and clinical research platform business that solves the 10-year challenge of new drug development through the power of technology and shortens the time it takes for new drug approvals, as well as a social networking service that connects pharmaceutical companies and patients, has begun disclosing clinical trial information to patients for Nippon Boehringer Ingelheim Co., Ltd. (Headquarters: Shinagawa-ku, Tokyo; Chairman and CEO: Jan Stefan Scheldt) using the platform of its clinical trial information disclosure media, “smt.” This initiative is a pioneering attempt to realize Patient and Public Involvement (PPI) in Japan, and will lead the way in improving transparency and efficiency in Japan’s clinical trial environment.
*1: PPI is an abbreviation for Patient and Public Involvement, which
refers to a system in which patients and the general public are involved not simply as “subjects” but as active partners in the planning, implementation, and information dissemination processes of clinical research and trials.
Background: Challenges of transparency and access to clinical trial information
In Japan, the disclosure of clinical trial information has not traditionally been an active area, and it has been difficult for patients and doctors not involved in the conduct of clinical trials to access information about trials they can participate in. From the perspective of PPI, there is an urgent need to create an environment in which patients and the public can actively participate in medical care.
This initiative: Collaboration with global companies
Nippon Boehringer discloses information in accordance with laws and industry voluntary standards, and by utilizing Buzzreach’s “smt,” a new channel has been opened that allows patients seeking clinical trial information to obtain it more easily than ever before. This will create an environment where patients and doctors not involved in clinical trials can easily access the treatment option of clinical trials, leading to concrete progress in PPIs.

https://www.searchmytrial.com/smt-sites/boehringer-ingelheim
Buzzreach’s track record and leadership
Buzzreach has supported the disclosure of clinical trial information for several major domestic and international pharmaceutical companies and university hospitals conducting clinical trials, and has established itself as a leading company in clinical trial information disclosure in Japan.In addition, with
an eye on the spread of decentralized clinical trials (DCT), the company is accelerating its growth as a “headwind-resistant infrastructure” that will build a unique Japanese clinical trial ecosystem, centered on clinical trial information disclosure.
Future outlook: Strengthening collaboration with university hospitals and cancer centers
Buzzreach will strengthen the clinical trial information disclosure infrastructure at large hospitals, such as university hospitals and cancer centers, which conduct many clinical trials, and promote efforts to increase fairness of patient access on a national level. This will be an important step in the realization of a Japanese version of DCT and in the growth strategy of the vertical startup.
About Nippon Boehringer Ingelheim
As a member of the Boehringer Ingelheim Group, headquartered in Germany, Nippon Boehringer Ingelheim conducts research, development, and supply of innovative pharmaceuticals in Japan. Its mission is to contribute to patients and society, and it aims to provide better treatment options.
About Buzzreach Inc.
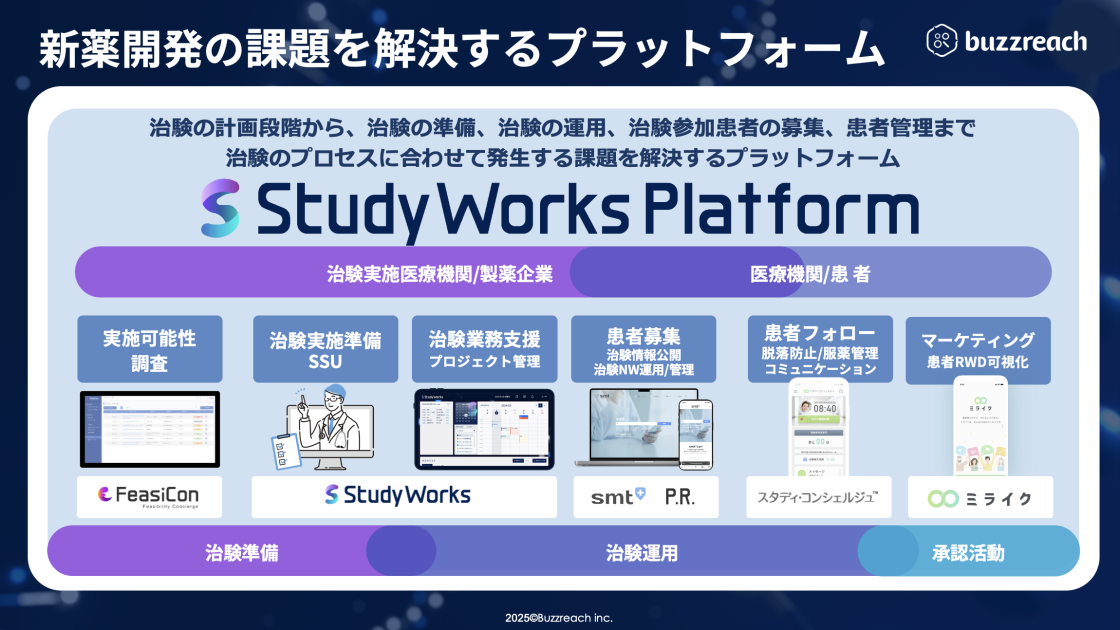
Buzzreach’s mission is to “use the power of technology to provide new options for as many patients as possible,” and they develop and provide a platform for streamlining clinical trial operations. Their main services include “StudyWorks,” the clinical trial information disclosure and management function “smt,” DCT-type subject recruitment using partner sites, and the patient-specific SNS service “Miraiku,” providing solutions that connect pharmaceutical companies, medical institutions, and patients.
▶ Government-promoted × Medical Institutions Favor Clinical Trial DX
The government has launched a series of policies related to the revitalization of new drug development, such as deregulation of GCP*1, the establishment of a clinical trial ecosystem*2, and the promotion of DCT, and has begun various initiatives to enable medical institutions conducting clinical trials to be involved in more clinical trials and clinical research more quickly.In DCT*3, the government is also supporting the development of a digital transformation environment necessary to make clinical trials a more familiar medical option, starting with e-recruitment, e-consultation, online medical consultations, visiting nurses, and easing delivery of investigational drugs to patients’ homes, pharmacies, and primary care physicians.
Medical institutions are also entering an era where they are being asked to “clarify management and supervision responsibilities” and “infrastructure that can accommodate various clinical trial formats while reducing the workload” in order to attract more clinical trials through digital transformation and operational efficiency improvements, as well as outsourcing clinical trial operations to partner sites in order to support DCT. As a result, various innovative movements are becoming more active at advanced medical institutions that conduct clinical trials.
In such an environment, with StudyWorks’ “unified management of clinical trial operations on a platform” and “unified management of partner site outsourcing operations compatible with the Japanese DCT” services, and clinical trial information utilizing SMT becoming more widespread and common as a treatment option, there are voices saying that “now is the best time to reform Japan’s clinical trial environment.” *
1 GCP: “Ministerial Ordinance on Standards for the Conduct of Clinical Trials of Pharmaceuticals” (GCP: Good Clinical Practice) established by the Ministry of Health, Labor and Welfare .
*2 Clinical trial ecosystem: A system led by the Ministry of Health, Labor and Welfare that efficiently conducts clinical trials through cooperation among all stakeholders, including pharmaceutical companies, medical institutions, regulatory authorities, and subjects, in order to quickly deliver therapeutic drugs to the public.
*3 DCT: Decentralized Clinical Trial is a new method for expanding clinical trial options. It is an initiative to improve the efficiency of clinical trials by working with cooperating medical institutions called partner sites, rather than consolidating all clinical trial operations at a single medical institution.
[Contact for inquiries regarding this matter]
Public Relations Officer, Buzzreach Inc.
Email: info@buzzreach.co.jp
URL: https://www.buzzreach.co.jp/contact
Company name: Buzzreach Inc.
Head office location: Minato-ku, Tokyo
Representative Director and CEO: Takateru Inokawa
Business: Provision of clinical trial and clinical research support SaaS, DCT support business, development and operation of patient recruitment platform utilizing partner sites, patient SNS business, etc.






