Notice of New Year’s holiday closure and thanks for the year
Press
We will be co-hosting a seminar and exhibiting a corporate booth at the 25th Conference on CRC and Clinical Trials 2025 in Omiya.

At the 25th Conference on CRC and Clinical Trials 2025 in Omiya, which will be held on September 14th (Sun) and 15th (Mon, national holiday), we will be co-hosting seminars and exhibiting at a corporate booth as detailed below.

This co-hosted seminar will focus on the theme of medical institution-led DCT, with Mr. Matsui of the Tokushukai Group Future Medical Research Center speaking on partner-site DCT utilizing the large-scale medical network unique to group hospitals. Next
, Mr. Uchino, head of IT at Buzzreach, formerly of the Digital Agency and the major gourmet information media company Retty, will discuss how “manufacturing” is carried out from the perspective of a system developer, and the theme of DCT platform development co-created with clinical trial sites, from the perspective of problem-solving and operational optimization from the perspective of medical institutions.
The panel discussion will also feature an impressive lineup of speakers, including Mr. Matsui of the Tokushukai Group Future Medical Research Center, the clinical trial site for this DCT project; Mr. Oyama of the Saiseikai Group; Mr. Mae of AstraZeneca, the clinical trial sponsor; Mr. Kaneko of IQVIA, the CRO; and Mr. Uchino, head of IT at Buzzreach, who will each share their perspectives.
At the exhibition booth, local bread novelties will be back for the first time in a long while! Also, with the theme of the AI agent that will be implemented in StudyWorks, we will be holding an event entitled “Experience! AI Agents” where you will be able to experience “what it would be like to have an AI agent support your everyday work.”
We look forward to seeing you at our booth.
■About the local bread novelty:
“Chichibu Gakukyu Koppepan” is a local bread made by the ” Chichibu Gakukyu Bread Center ” in Chichibu City, Saitama Prefecture. While they primarily make bread for school lunches, they also make products for the general public, which can be purchased at supermarkets in Chichibu City.




■ Details of the co-hosted seminar Seminar title:
Medical Institution-led DCT will change the structure of clinical trials in Japan – Towards a future standard co-created by medical institutions and sponsors –
Details: Sunday, September 14th, 12:20-13:20, Venue 2 (Sonic City Hall, 2nd floor small hall)
Chair ①: Dr. Kento Asano, Clinical Research Center, Department of Future Medical Development, Osaka University Hospital
Chair ②: Takateru Inokawa, CEO, Buzzreach Inc.
Speaker 1 (lecture/panel): Ms. Yumi Matsui, Clinical Trial Promotion Department, Business Promotion Headquarters, Future Medical Research Center, Tokushukai Group
Speaker 2 (lecture/panel): Tomoaki Uchino, General Manager, IT Headquarters, Buzzreach Inc.
Speaker 3 (panel): Ms. Yuko Mae, Clinical Development Department, Research and Development Headquarters, AstraZeneca K.K.
Speaker 4 (panel): Mr. Shintaro Kaneko, Site Solutions Division, IQVIA Site Solutions Japan LLC
Speaker 5 (panel): Mr. Akihiro Oyama, Joint Clinical Trial Promotion Office, Saiseikai, Social Welfare Corporation, Imperial Gift Foundation
| Topic 1: The challenge of large-scale DCT implementation – The reality of preparations and problem-solving faced by the field |
In recent years, the environment surrounding clinical trials has changed rapidly, and patient-centered and efficient clinical trial models are being sought. In this context, Tokushukai Group is working to introduce “Partner Site Type DCT (Decentralized Clinical Trial)” in cooperation with clinical trial sponsors, utilizing the large-scale medical network that only group hospitals can offer.
In the past, when conducting clinical trials at multiple facilities, it was necessary to set up many facilities to accumulate cases, which caused major issues such as the burden of coordinating with the parties involved and the CRO costs.This time, Tokushukai Group is taking advantage of the network and collaboration system between hospitals or facilities to build a DCT scheme that limits the number of facilities in the same area, and is challenging to implement a new model that will contribute to cost reduction for the sponsor of the clinical trial.
Furthermore, to address the unique operational management and information sharing requirements of DCT, we have introduced a new digital platform initiated by the medical institution, significantly reducing the resulting complex workload and management effort. This seminar will explore how we have utilized digital technology to create a patient-friendly environment while minimizing the workload for physicians and CRCs. We will also discuss the preparation process, challenges, and practical efforts leading up to the launch of the system, from a medical institution’s perspective. In particular, we will discuss the coordination and operational challenges we faced in collaborating with multiple partner institutions and the solutions we used to resolve them. This project, based on the sponsor’s goals of “improving case accumulation” and “moving away from the traditional clinical trial implementation model,” is also an effort to realize a new clinical trial ecosystem that goes beyond institutional frameworks such as single IRBs and FMVs. As a medical institution, this challenge marks the first step in our efforts to collaborate more closely with sponsors and co-create a sustainable and valuable clinical trial model. We hope this seminar will provide practical tips and insights for medical institutions and stakeholders considering the implementation of DCT.
| Topic 2: DCT Platform Development in Collaboration with Clinical Trial Sites – The Challenge of Problem-Solving and Operational Optimization from Medical Institutions’ Perspectives |
To realize a functional DCT operation on-site, it is essential not only to introduce technology, but also to design and implement it from the perspective of “whether the medical institution (site) can actually use it.” As an IT vendor, we were responsible for developing and supporting the implementation of a digital platform to reduce on-site operational burden and improve the efficiency of information sharing in the medical institution-led DCT implementation promoted by the Tokushukai Group.
In this session, we will share our process of not simply providing a system, but also constantly asking ourselves, “What is a digital tool that fits into actual workflows?” in close collaboration with medical institutions. In particular, we will introduce examples of how we designed a system that functions at a practical level and implemented it on-site in a short period of time to address requirements unique to DCT
, such as “inter-site collaboration” and “remote patient support,” which differ from traditional clinical trials. While digital implementation often does not necessarily translate into on-site improvements, we hope to share the “digital strategy for DCT establishment” that we co-created with the site and provide insights for those considering DCT implementation in the future.
| Panel discussion: Co-creation of Japanese-style DCT between medical institutions and clients – How to create an environment for future standards – |
This panel discussion will focus on the Japanese-style DCT, which is operated primarily by medical institutions, and will discuss how this DCT model was created from the perspectives of clinical trial institutions, partner sites, clinical trial sponsors + CROs, DCT platformers (IT vendors), and patients. Moderators will be Professor Asano of Osaka University, who has experience in launching a full DCT model with 2,000 cases at one facility, and Inogawa of Buzzreach, who will share the goals, challenges, and discoveries of each stakeholder through the project.
■About the exhibition booth:
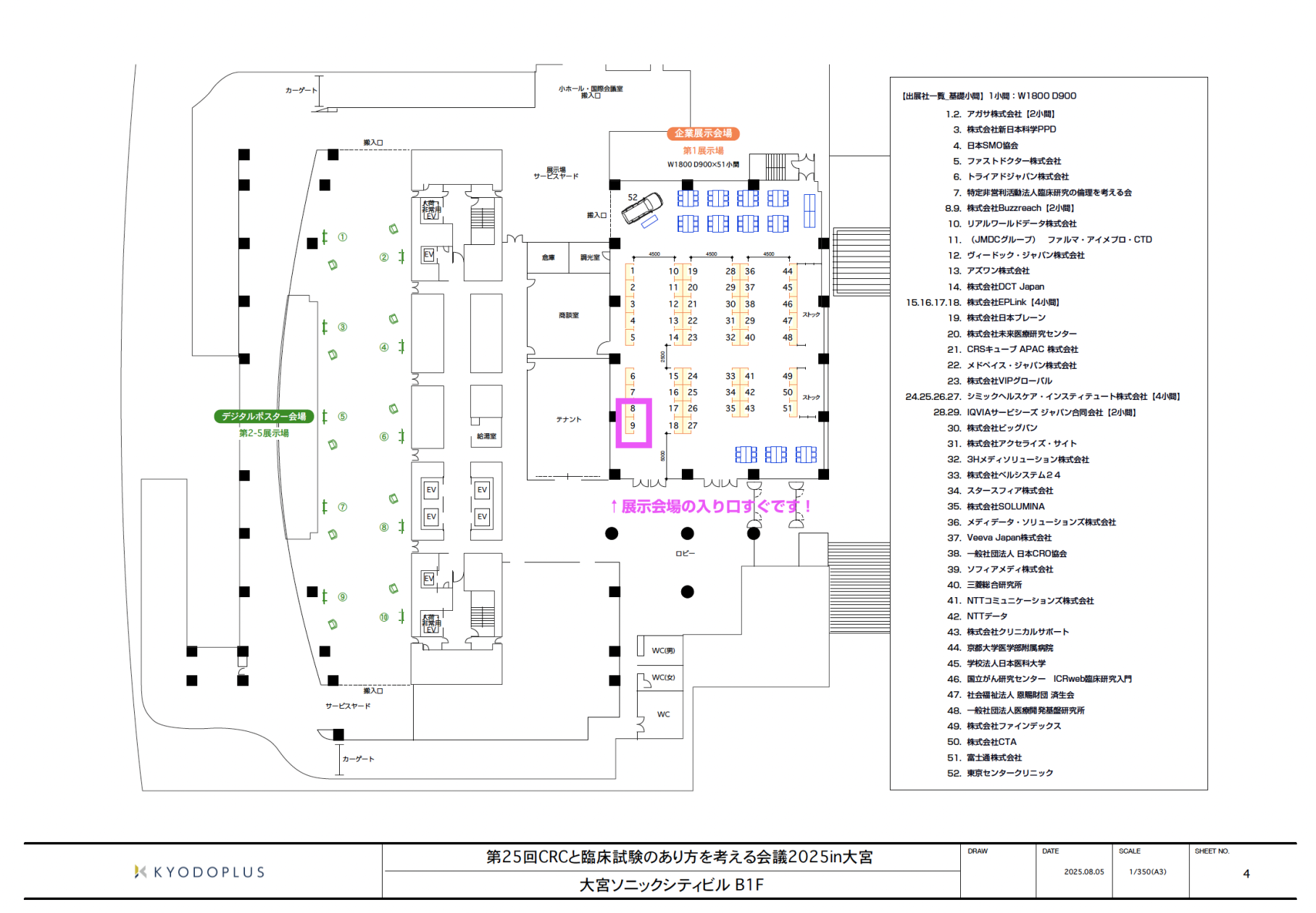
Event details
Conference name: 25th Conference on CRC and Clinical Trials 2025 in Omiya ( https://www.ctpf.or.jp/crc2025/ )
Dates: Sunday, September 14th and Monday, September 15th, 2025 (national holiday)
Venue: Omiya Sonic City 1-7-5 Sakuragicho, Omiya-ku, Saitama-shi, Saitama 330-0854
Conference representative: Tomomi Hata (National Cancer Center Hospital)
Theme: The future of CRC and clinical trials – internationalization and professionalism
Number of participants: Approximately 2,800
Organizers: Clinical Trial Support Foundation
Supporters: Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare
Organizing secretariat: Kyodo Plus Co., Ltd. 20-110 Tatsumi, Kita-ku, Okayama-shi, 700-0976
TEL: 086-250-7681 FAX: 086-250-7682 Email: crc2025@kwcs.jp