Services

Product Overview
-

Streamlined Project Management for Clinical Trials and Research

Study Works is a clinical trial management tool born from the voices of CRCs (Clinical Research Coordinators) and CRAs (Clinical Research Associates). It enables one-stop management of clinical trial operations, centralizing the vast management tasks generated within trial sites through the functions of Study Works. This reduces the workload by allowing seamless management of stakeholders involved in clinical trials, such as pharmaceutical companies, CROs (Contract Research Organizations), patients, and other departments within trial sites.
-

Subject recruitment support

The problem with many clinical trials is the inability to find patients who can participate in them.This inability to find patients means that the trial period must be extended and the number of medical institutions conducting the trial must be increased.Extending the trial by six months would incur additional costs of approximately 200-300 million yen.P.R. (Patient Recruitment) mainly uses partner sites to approach patients attending medical institutions surrounding the clinical trial institution, and can also access a database of approximately 2.5 million people seeking clinical trials through various media, patient groups, and collaborative partner companies. Using these services, you can effectively gather patients who can participate in clinical trials, achieving case accumulation and clinical trial efficiency.
-
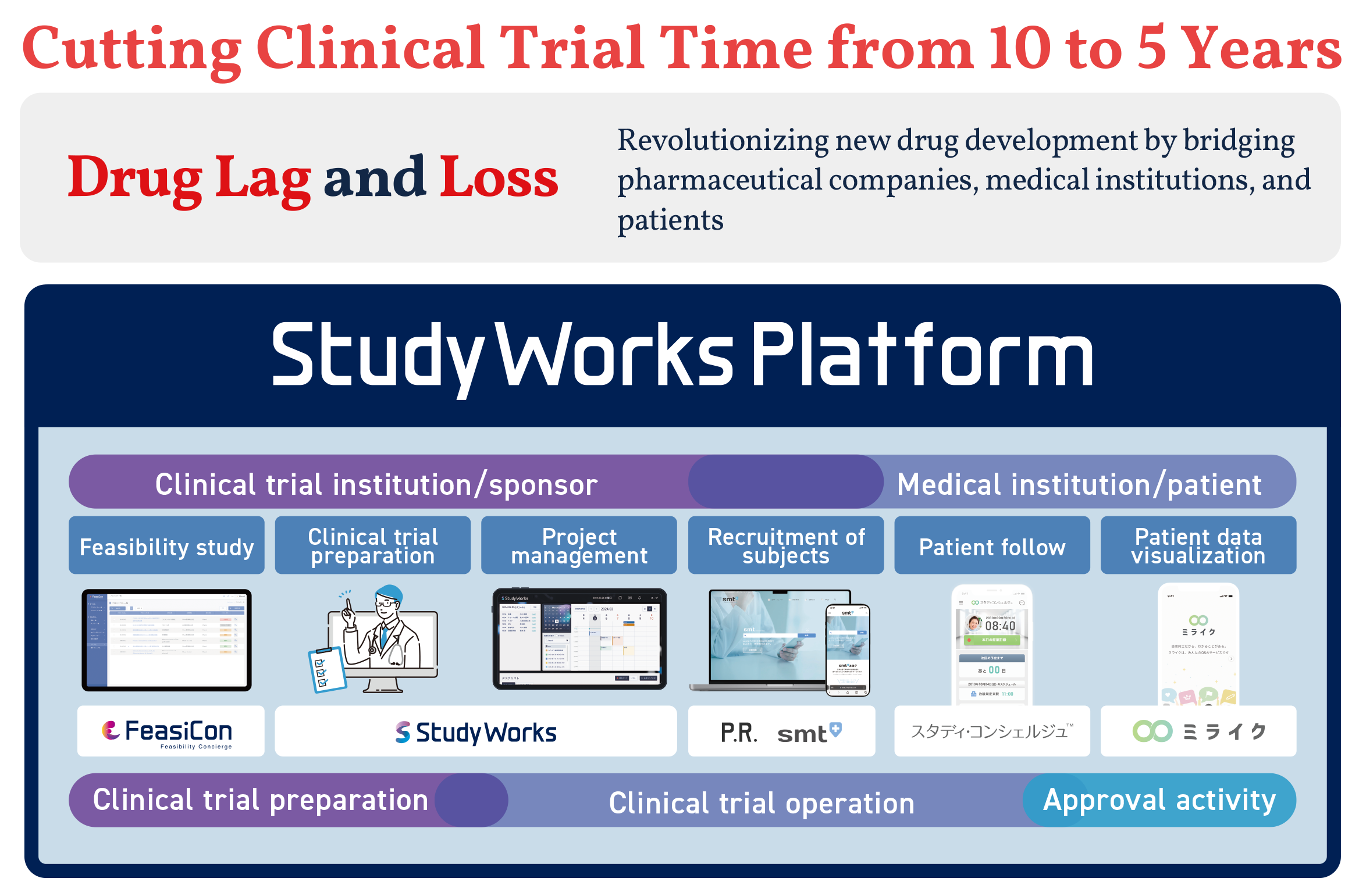
Clinical Trial Information Sharing and Management

This function allows pharmaceutical companies and trial sites to register, share, and manage clinical trial information. It provides information to patients and their families who need clinical trial information. It also has a system that matches trial sites with patients and allows for online participation applications. The system can integrate with various organizations such as PRO, advocacy groups, and media, making it a comprehensive tool for diverse patient recruitment.
-

smt Solutions Suite

This initiative utilizes the smt platform to support smooth public information disclosure by university hospitals and other clinical trial facilities and pharmaceutical companies as part of providing information on treatment options to patients and doctors (medical institutions) not involved in clinical trials, from the perspective of PPI (Patient and Public Involvement) and patient-centricity.
-
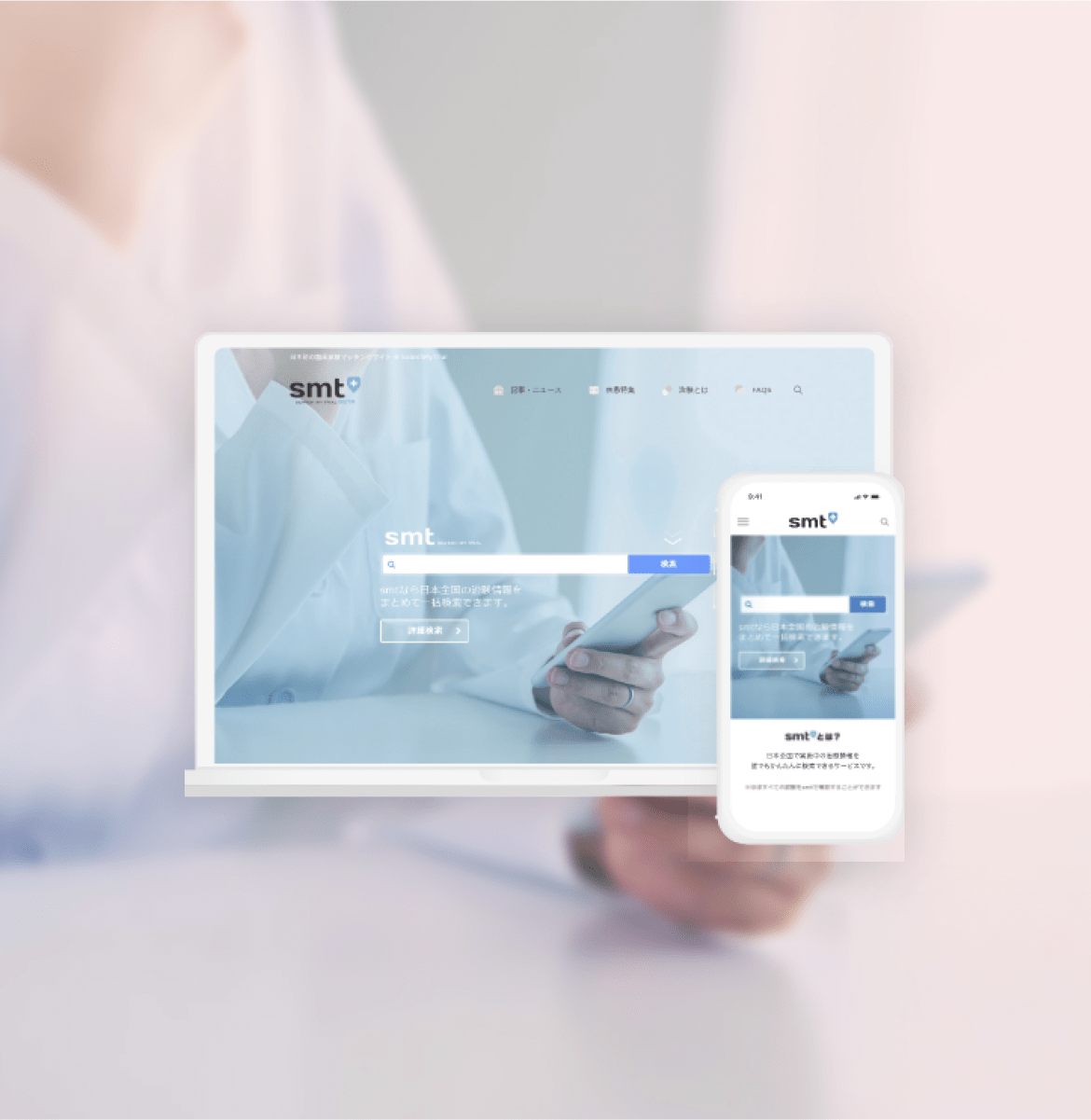
Clinical Trial Management and Retention App

This cutting-edge clinical trial app for participants acts as a virtual assistant for Clinical Research Coordinators (CRCs), delivering medication reminders, visit notifications, and alert messages to trial patients. By leveraging the app's communication and information-sharing capabilities, it significantly reduces the risk of patient dropouts from clinical trials. Moreover, the app features an integrated survey tool, making it a versatile solution with proven usage as an ePRO (electronic Patient-Reported Outcomes) platform.
-

Feasibility and Site Selection

FeasiCon (Feasibility Concierge) is a support system that enables the centralized management of feasibility studies to site selection for clinical trials and clinical research. The system allows the entire process—from survey requests to data aggregation and site selection—to be completed online. After the survey and trial implementation, site information can be stored in a database.Since English is supported, feasibility studies and site selection can be conducted both domestically and internationally.
-
Patient SNS

This dynamic community allows patients to share information and offer advice on physical and emotional concerns, as well as illnesses, with others who are in similar situations. Miilike’s concept is to "connect with like-minded individuals to support and uplift each other together."